Implantable left ventricular assist device (LVAD) therapy is used to improve quality of life, alleviate symptoms and extend survival rates in patients with advanced heart failure. Patients with LVADs require chronic anticoagulation to reduce the risk of thromboembolic complications, and they commonly experience bleeding events.
The Problem
LVAD patients require chronic anticoagulant therapy to reduce the risk of thromboembolic complications. Anticoagulation management in patients with LVADs continues to be a challenge. Patients and their clinicians are faced with the daily challenge of needing stable and predictable anticoagulation versus the bleeding risks and other serious side effects that are associated with this anticoagulation treatment. Warfarin, the most commonly used oral anticoagulant for LVADs, is known to be a difficult medication to manage due to its unstable, narrow therapeutic window, and many drug-drug interactions.
There is a need for a new, superior and safer VKA anticoagulant for LVAD that improves outcomes and relieves patients and their healthcare providers from some of warfarin’s greatest challenges.
LVAD SYSTEM
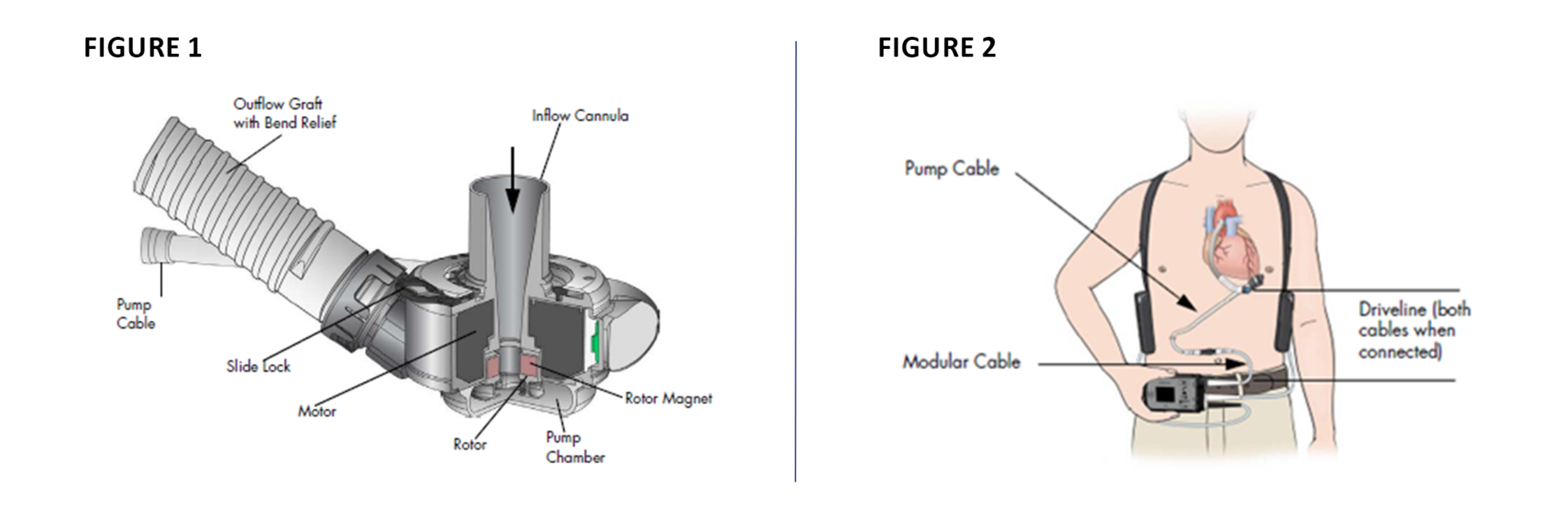
FIGURE 1 illustrates the left ventricular assist device (LVAD) blood pump components which include the inflow cannula, a lower housing, a screw ring to attach the pump cover to the lower housing, a motor, the outflow graft, and a pump cable.
FIGURE 2 illustrates the LVAD system comprising the Driveline, composed of a pump cable that extends from the LVAD through the skin, and the modular cable which connects the pump cable to the system controller. The driveline powers the pump motor and facilitates communication with the system controller.
THE PREVALENCE
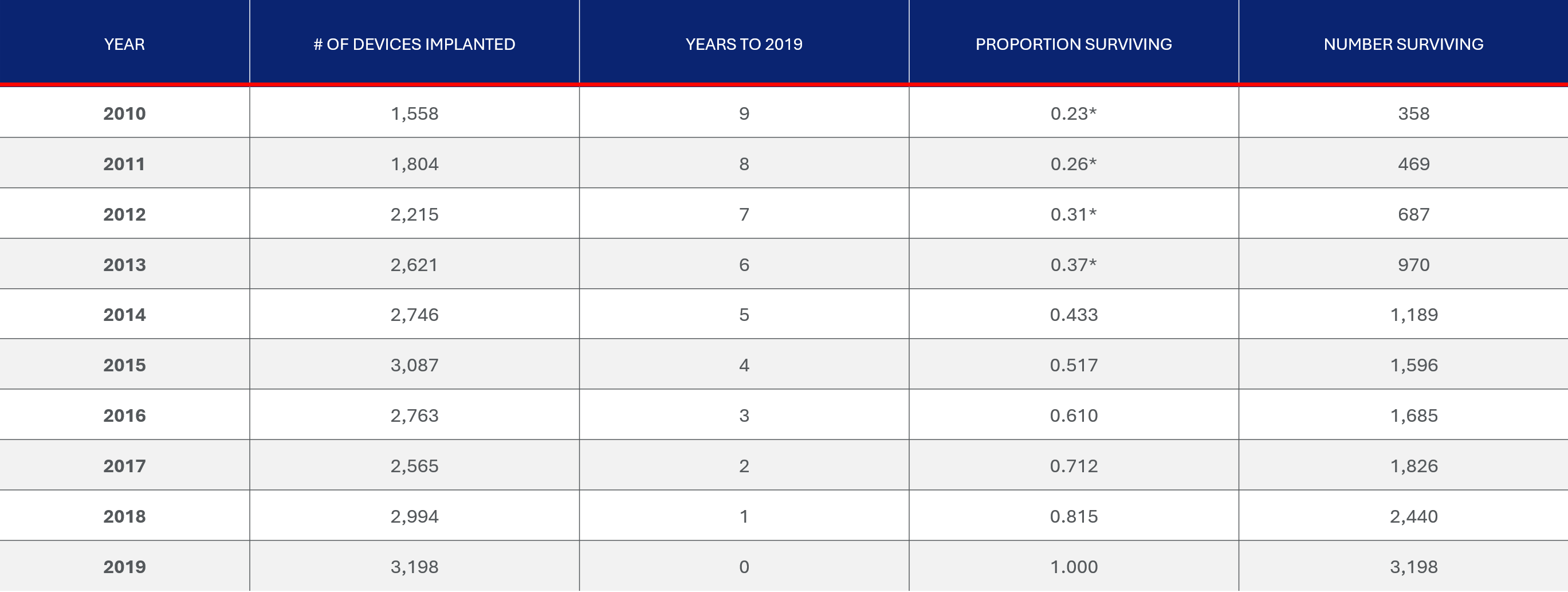
The INTERMACS report provides number of implanted devices by year from 2010 to 2019 as well as the survival time. The chart below provides a summary of the expected number of subjects surviving in 2019. The total number of subjects surviving in 2019 from implantations beginning in 2010 is approximately 14,419.
PREVALENCE
The INTERMACS report provides number of implanted devices by year from 2010 to 2019 as well as the survival time. The total number of subjects surviving in 2019 from implantations beginning in 2010 is approximately 14,419.
Our Solution
Our new vitamin K antagonist anticoagulant, tecarfarin, is designed to overcome the challenges of warfarin, prevalent serious side effects and cumbersome dosing, and provide superior and safer anticoagulation.
Tecarfarin is metabolized via a different pathway than warfarin and data shows that its efficacy is unaffected by common drug-drug interactions or kidney impairment, which are common in these patients.
Phase 2/3 clinical trials show that tecarfarin, compared to warfarin, may offer superior stability and time in therapeutic range that inversely correlates with major events.
Drugs in newer classes such as direct oral anticoagulants (DOACs) are not recommended for patients with implanted cardiac devices, leaving tecarfarin the only new anticoagulant being studied for these underserved patients.